what do neuropeptides do?
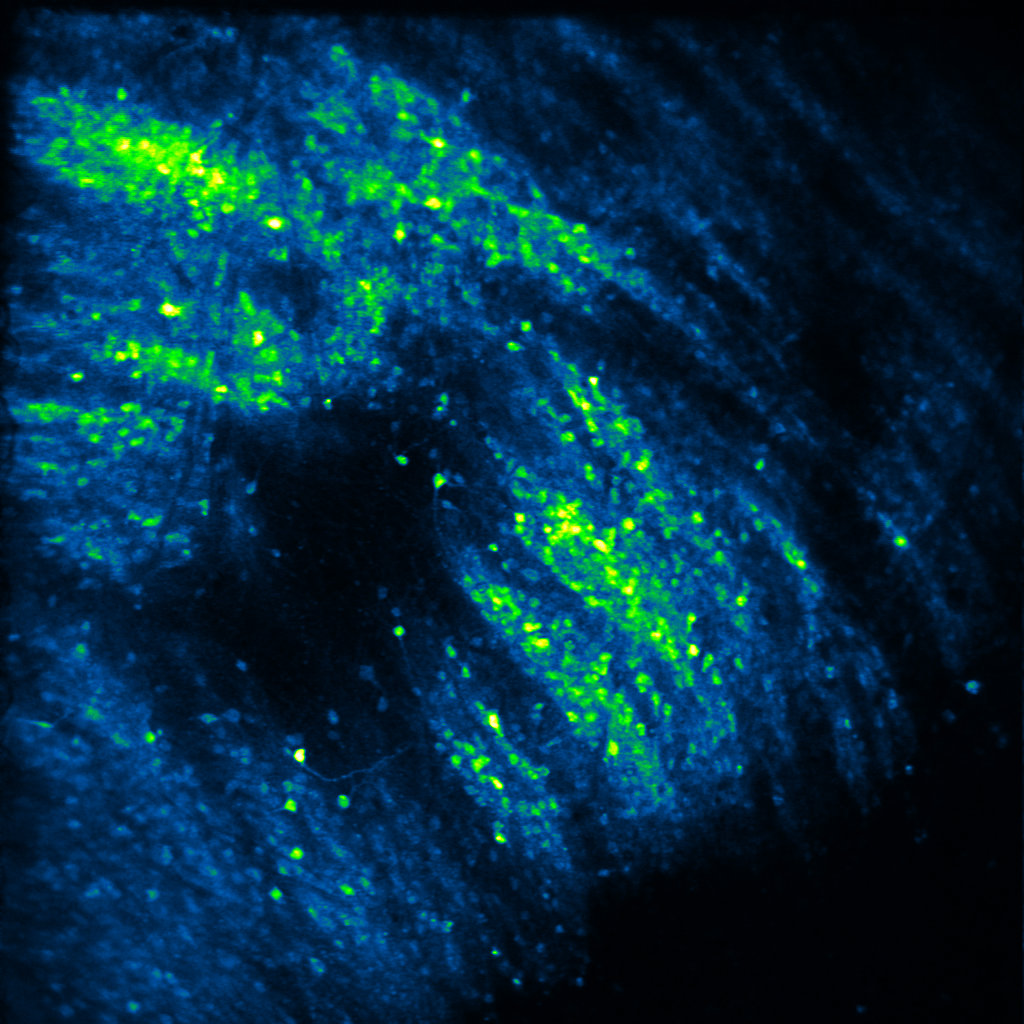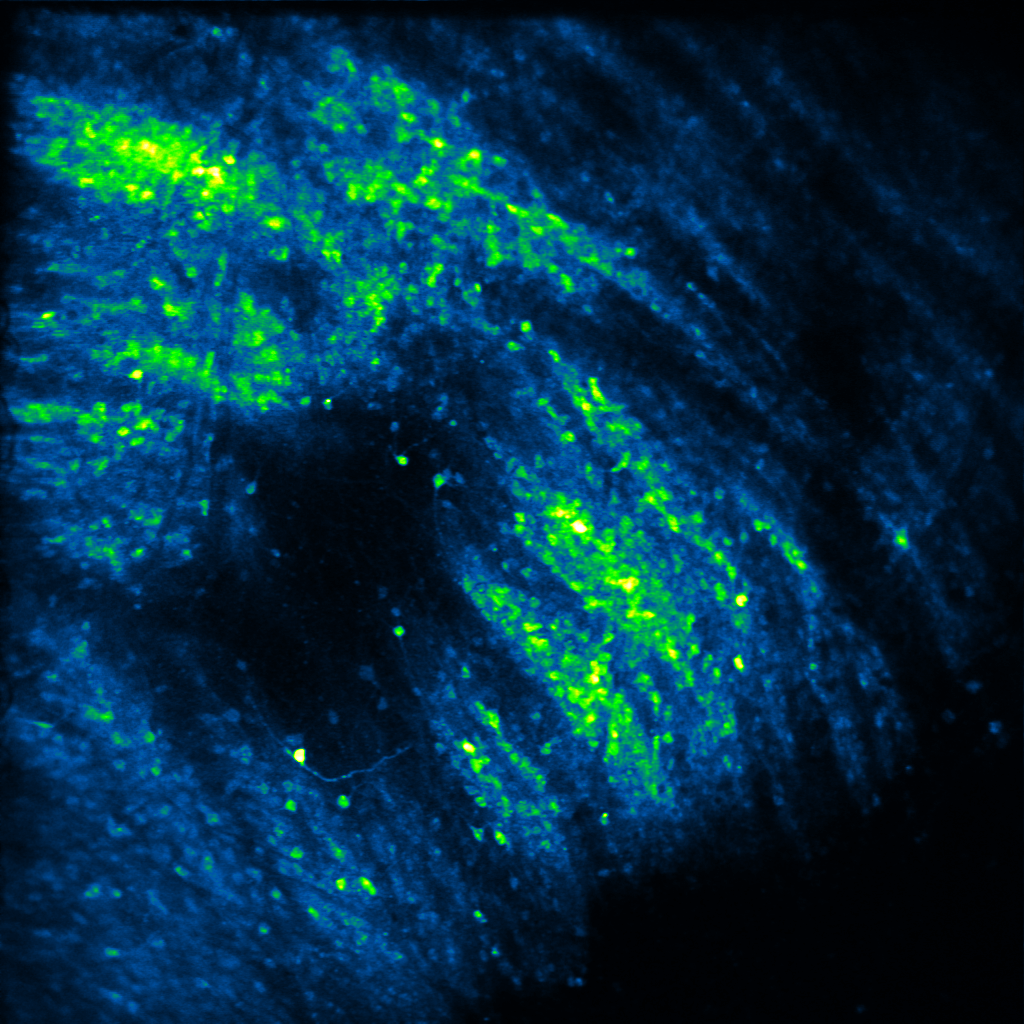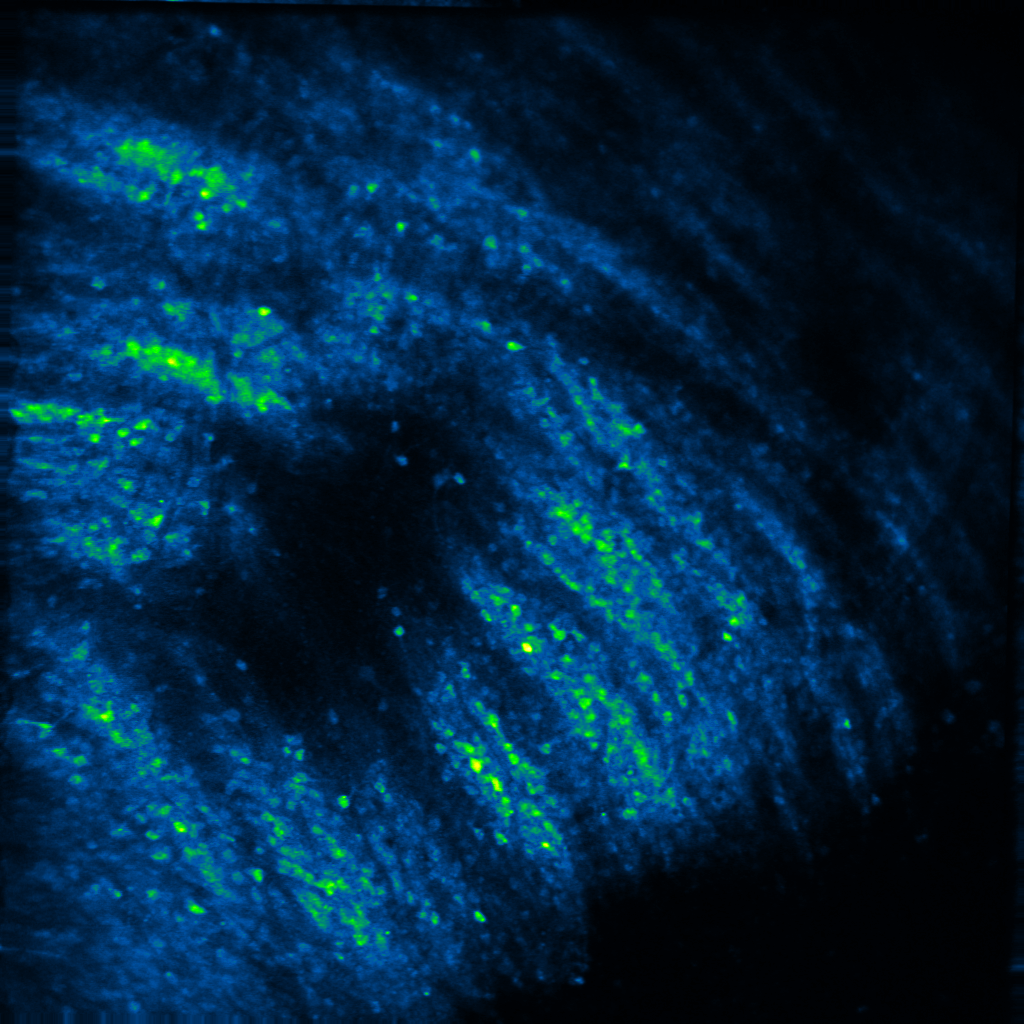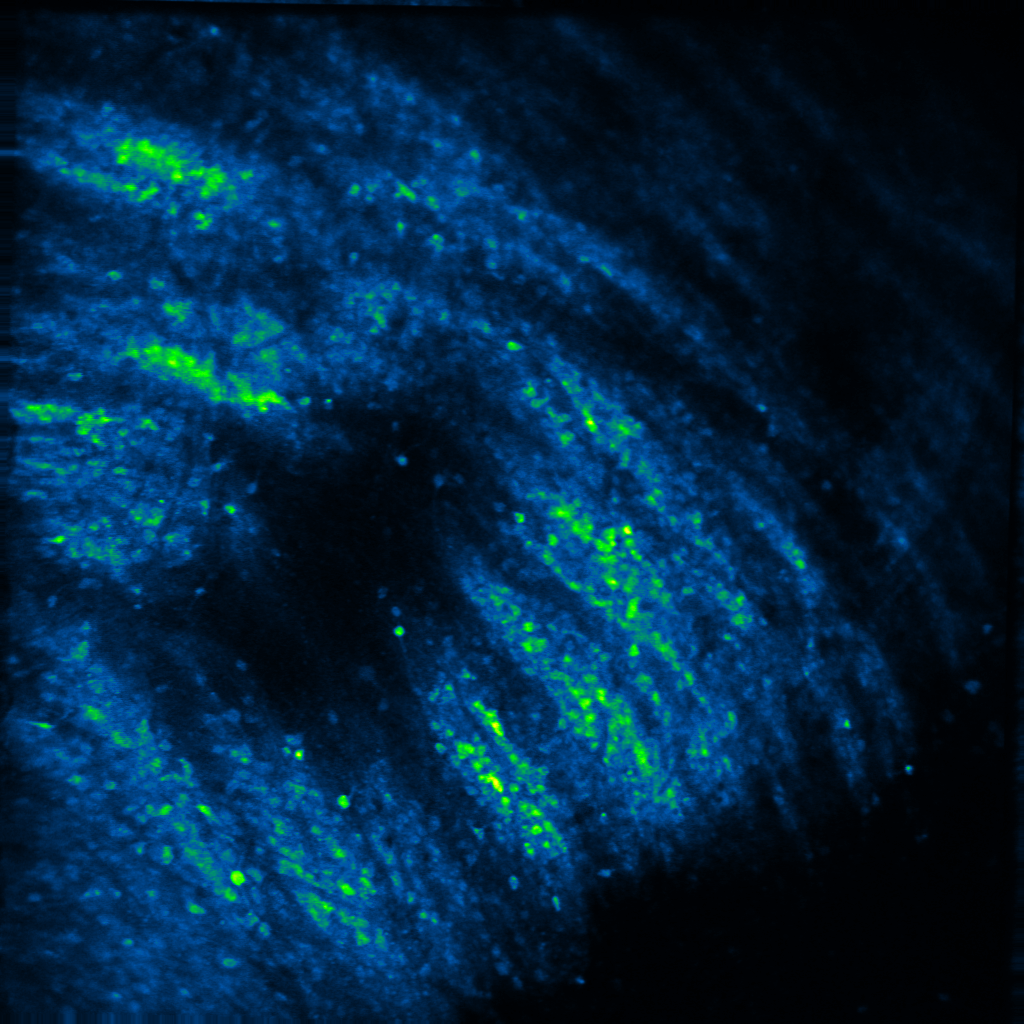
Decades of elegant work has shown that neuropeptides can powerfully modulate affective states, and thus, behavior. Yet, how they do so is unclear. In his postdoctoral work, Raaj showed that the neuropeptide dynorphin can sculpt neuronal activity of afferent glutamatergic circuits from the basolateral amygdala in the striatum. This research was the first to demonstrate that neuropeptide signaling modulates motivated behavior via a feed-forward inhibitory mechanism at both slow and fast timescales. In the Gowrishankar Lab, we aim to study how dynorphin signaling controls neuronal activity in the basal ganglia, the amygdala and hippocampus to promote motivated behavior utilizing genetic mouse models and systems neuroscience techniques such as viral tracing, fluorescent in situ hybridization, in vivo two-photon calcium imaging, in vivo fiber photometry and optogenetics.
what are the rules that govern neuropeptide release?
Decades of work has suggested that neuropeptides are released to signal a shift in an internal state via sustained neuronal activity. For example, dynorphin is suggested to be released during stress or high arousal, acting as a break upon motivational drive. Yet, measuring neuropeptide dynamics and pinpointing a causal role for them has been elusive. In recent work from the Bruchas and Tian labs, Raaj characterized a suite of novel opioid/neuropeptide biosensors, and showed dynorphin dynamics during motivated behavior at both slow and fast timescales. In the Gowrishankar Lab, we aim to study how coordinated dynorphin dynamics across multiple brain regions act as "neuropeptide hubs” that gate internal states, thereby promoting motivated behavior. To do so, we utilize genetic mouse models and newly developed genetically-encoded biosensors and actuators in conjunction with in vivo two-photon calcium imaging, in vivo fiber photometry and optogenetics.
how do animals acquire, maintain and flexibly adapt motivated behavior?
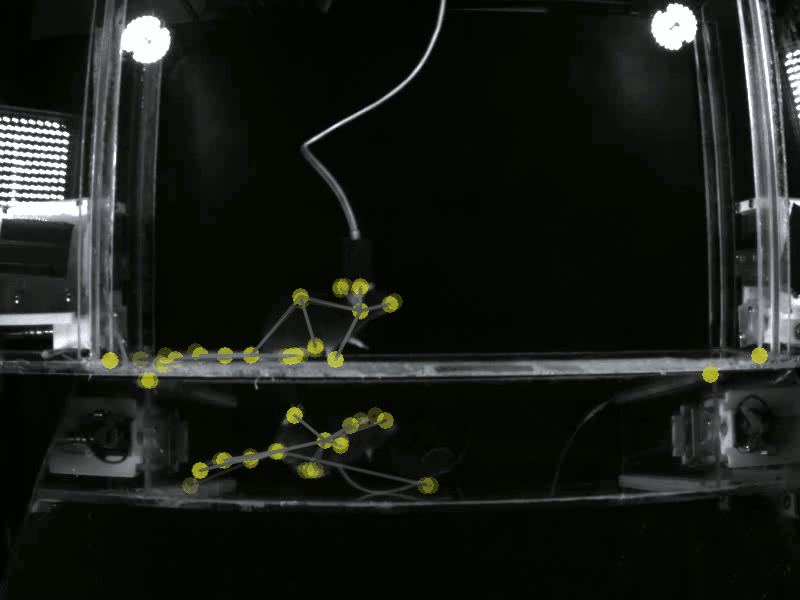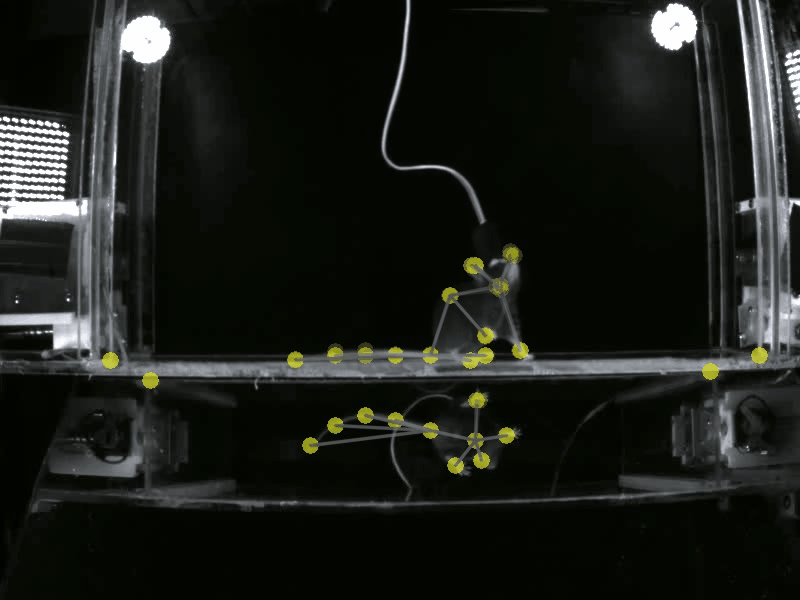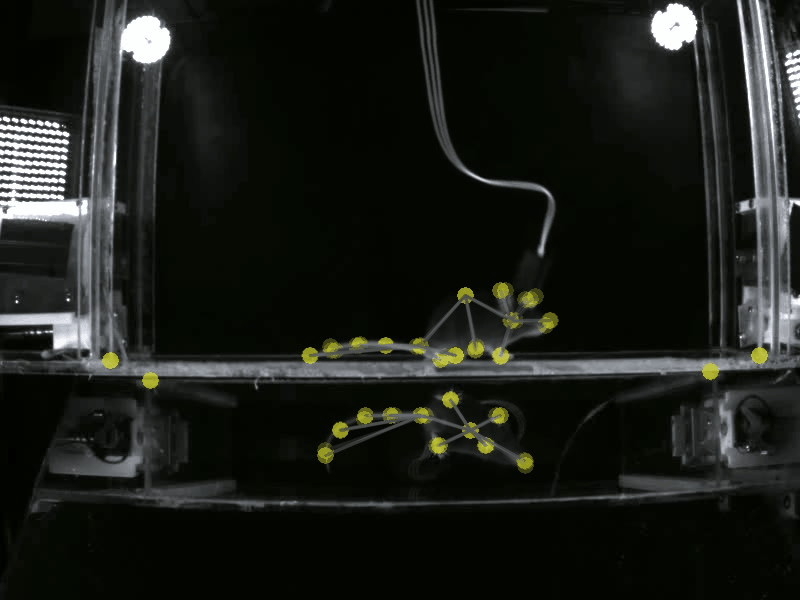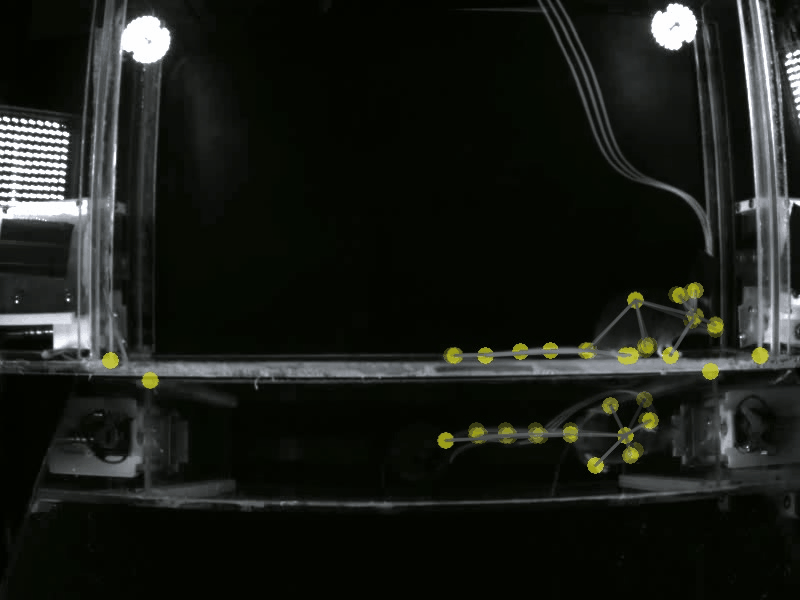
Prior work has shown that there are individual differences in how animals acquire motivated behavior. Yet, a deeper understanding of the behavioral variables that govern this acquisition, and the neural substrates that may differentially modulate these variables is unclear. A deeper understanding of these fine-grained behavioral dynamics is particularly necessary as we attempt to model how these mechanisms may go awry in neuropsychiatric disorders, such as substance use disorders. Thus, in the Gowrishankar Lab, we aim to delineate individual differences in how animals acquire, maintain and adapt their motivated behaviors using SLEAP-based pose estimation methods and artificial neural network modeling. Coupled with our expertise in monitoring neural circuit activity, our work will uncover novel behavioral variables and neural activity correlates that predict the transition to maladaptive, persistent substance use.